Understanding Electrochemically Activated Persulfate and Its Application to Ciprofloxacin Abatement
Abstract
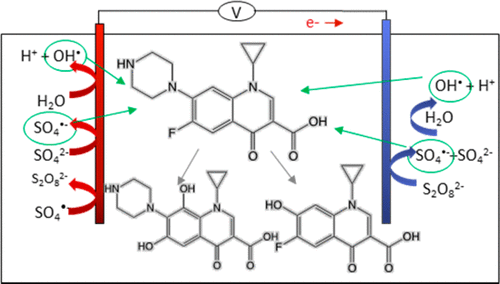
This study offers insight into the roles anodic and cathodic processes play in electrochemically activated persulfate (EAP) and screens EAP as a viable technique for ciprofloxacin degradation in wastewater. Sulfate radical formation at a boron-doped diamond (BDD) anode and persulfate activation at a graphite cathode were experimentally elucidated using different electrolytes and electrochemical setups. Rapid ciprofloxacin transformation occurred via pseudo-first-order mechanisms with respect to ciprofloxacin in persulfate electrolyte, reaching 84% removal in 120 min using EAP. Transformation pathways were compared to those in nitrate and sulfate electrolytes. Ciprofloxacin removal rates in the electrochemical system were 88% and 33% faster in persulfate than nitrate and sulfate electrolytes, respectively. Total organic carbon removal rates were 93% and 48% faster in persulfate than nitrate and sulfate, respectively. Use of sulfate electrolyte resulted in removal rates 6–7 times faster than those in nitrate solution. Accelerated removal in sulfate was attributed to anodic sulfate radical formation, while enhanced removal in persulfate was associated with cathodic persulfate activation and nonradical persulfate activation at the BDD anode. Quenching experiments indicated both sulfate radicals and hydroxyl radicals contributed to degradation. Comparisons between platinum and graphite cathodes showed similar cathodic persulfate activation and ciprofloxacin degradation.